)
TRVST
TRVST
Safer Medicines for the World
We are the global leaders in the design and provision of software to support medicine verification and traceability. Every day, our systems check the authenticity of millions of packs of medicine before they are supplied to members of the public, detecting falsified and substandard products, combatting medicine diversion and preventing harm to patients.
The Global Solution
Governments around the world work tirelessly to protect their citizens from the scourge of falsified medicinal products. This illicit industry is worth tens of billions of dollars annually and, according to the WHO, affects one in ten medicinal products in developing countries.
Emerging from the international response to the Covid-19 pandemic, TRVST is the world’s first global medicines verification system. Falsified drugs contribute to treatment failure, adverse drug reactions and increased drug resistance, especially in the case of vaccine, antiretroviral, antimalarial and antibiotic medications in low- and middle-income countries.
TRVST combines verification with traceability to increase visibility across the pre-country pharmaceutical supply chain and promote authentication at the national level. It supports the needs of governments, medicine regulatory authorities, law enforcement agencies and healthcare providers. It promotes the adoption of regulatory standards, best practices and builds public confidence in medicine safety and healthcare programmes.
TRVST is the brainchild of the Verification and Traceability Initiative (VTI) set up by stakeholders including UNICEF, Gavi, the Bill & Melinda Gates Foundation, the Global Fund, USAID and the World Bank. Working in partnership with national medicine regulatory authorities in Nigeria and Rwanda, the VTI commissioned Solidsoft Reply to apply their expertise and experience to deliver a system that is simple to use, broad in scope and effective in detecting falsification and diversion, wherever it occurs.
)

Verification and Traceability Initiative

What is Medicines Verification?
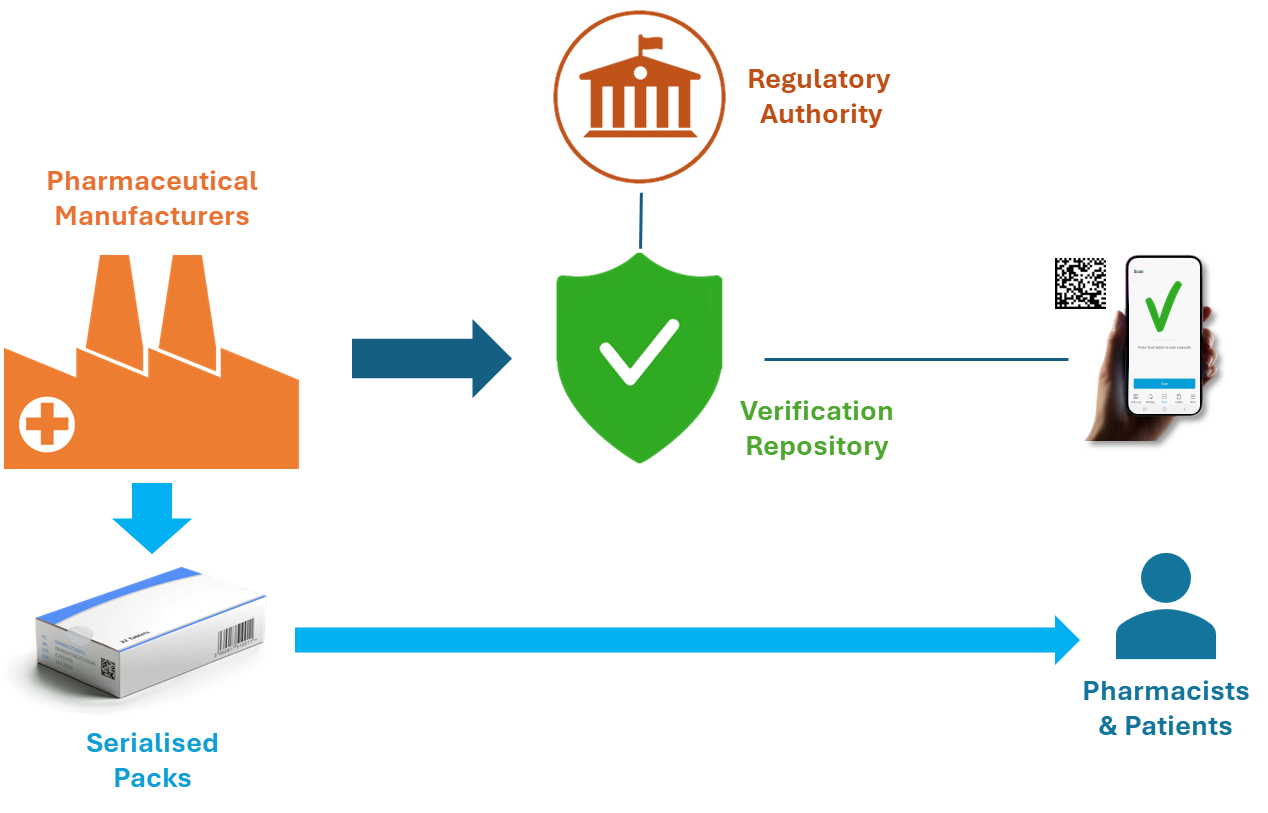
Pharmaceutical manufacturers around the world increasingly serialise their medicinal products. They provide unique identifiers for each pack of medicine that they place in the market. These identifiers are printed as two-dimensional barcodes, often following GS1 healthcare standards. By uploading their serial numbers to a trusted repository, manufacturers enable pack verification at any location throughout the supply chain or beyond, including the point of dispense, in healthcare institutions or even in the patient’s own home.
By scanning the barcode, clinicians and patients increase their confidence in the authenticity of each pack of medicine. If a unique identifier fails the test, alerts help manufacturers identify potential falsification or diversion and provide visibility to regulatory authorities. If the pack has expired, or if the batch has been recalled, this is immediately reported to the user, ensuring that substandard products are never used.
The unique identifier creates a ‘digital twin’ for each commissioned pack, allowing it to be tracked across the supply chain until it is supplied or decommissioned. This allows the provenance of each pack to be determined and helps to detect unusual movements or events that may signify falsification or diversion. By integrating with other systems, the verification system can support other needs such as closed-loop medicine management in healthcare institutions, national pharmacovigilance and detection of reimbursement fraud.
GS1 Standards
We are committed to interoperability across the pharmaceutical and healthcare sectors. We are proud partners of GS1 and support their standards in all we do. GS1 shares our passion for patient safety in healthcare and provides the barcode specifications that power modern medicine verification approaches, together with the track and trace standards that enable supply chain visibility and automation around the world.
The innovation never stops. In recent years, GS1 has introduced Digital Link technology to extend their identifiers across the web, enable new scenarios and streamline transactions across the supply chain. Solidsoft Reply provides Digital Link resolution services alongside our other verification and traceability systems to support electronic patient information and other uses.

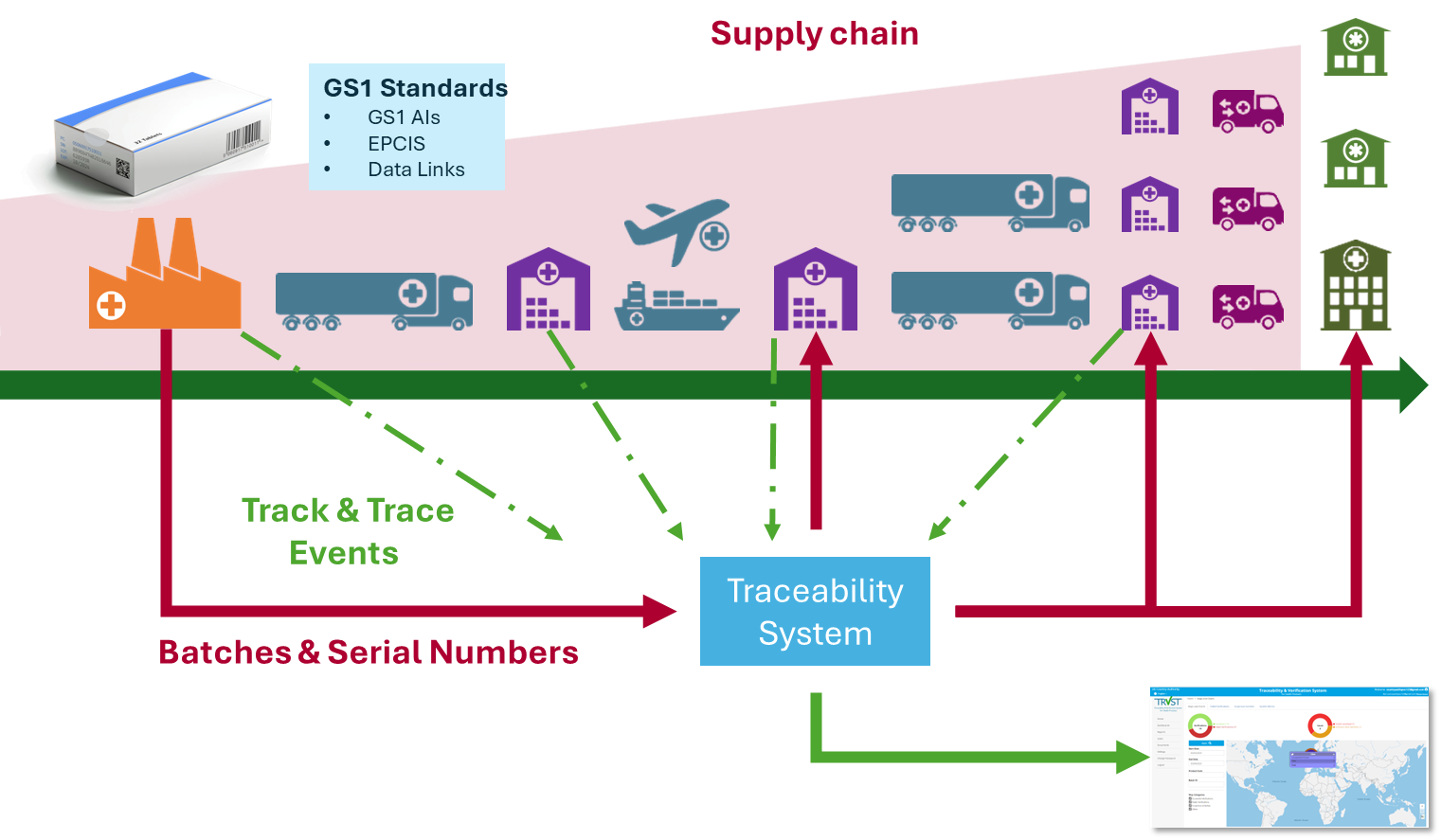
What About Traceability?
When it comes to medicine verification, we approach it on two fronts. Firstly, there's the straightforward authentication of each medicinal pack using a unique identifier. Pharmacists and clinicians simply scan a barcode to ensure the pack's authenticity before dispensing it to patients. It's a simple yet effective model, offering significant assurance to both healthcare providers and patients with minimal investment.
But we don't stop there. We delve deeper into the complexities of the pharmaceutical supply chain. Falsification and diversion can occur at any point, sometimes completely bypassing the legitimate channels. That's where traceability comes in, adding another layer of insight into the origin and history of each pack. By tracking its journey from manufacturing to distribution, we build a comprehensive record of its lifecycle. This not only enhances assurance but also provides valuable intelligence to combat falsification and diversion.
We advocate for traceability of medicinal products by employing modern industry standards such as GS1 EPCIS and related specifications. This not only offers greater visibility and insight but also ensures our systems stay in line with evolving regulatory requirements worldwide.
Electronic Product Information
When it comes to safe use of medicines, verifying the authenticity of medicinal packs is just one part of the puzzle. Another part is the provision of accurate and up-to-date product and patient information in the user’s language.
We make it easy for clinicians and members of the public to obtain this information electronically. By scanning the same barcode used to verify the authenticity of the pack, users download the correct product or patient information in the language of their choice and view it directly on their device.
We use GS1 Digital Links to enable this. Digital Links connect each physical pack of medicine to the wider web, making it easy to get the right information and services to the clinician or patient at the press of a button, helping them to use medicines effectively and safely.

Further Reading

Solidsoft Reply
)
Solidsoft Reply is a leading technology company creating award-winning solutions utilising the Microsoft Azure cloud platform. As a globally acclaimed Microsoft AI Cloud Solutions Partner, we specialise in GS1 traceability systems worldwide, crucially ensuring the authenticity, legality, and safety of our customers’ products and services. Serving non-profits, NGOs, healthcare, and the pharmaceutical industries, we deliver technology for positive social impact. Your products, safe in our hands.